First Aid & OTC Products
Find a Distributor
We sell our products through our website by the case and through authorized distributors across the U.S. and around the globe.
Shop Safetec’s First Aid & OTC products for your facility online by the case, or if you’re looking for larger quantities at better pricing, allow our team to see if you qualify to become a distributor.
- COVID-19
- First Aid
- Miscellaneous
What percentage of alcohol does hand sanitizer need to be effective?
What is the alcohol content of your hand sanitizer?
Safetec Instant Hand Sanitizer contains 66.5% ethyl alcohol, which meets the CDC recommendation to use a hand sanitizer with at least 60% alcohol in the absence of soap and water.
Why can’t I find your product on the EPA List N: Disinfectants for Use Against SARS-CoV-2?
This EPA list only contains the primary registrant’s product name and EPA registration number. All of our surface disinfectants are sub registered products, so you will not be able to find them by name on this list. According to the EPA, “The easiest way to find a product on this list is to enter the first two sets of its EPA registration number into the search bar below. For example, if EPA Reg. No. 12345-12 is on List N, you can buy EPA Reg. No. 12345-12-2567 and know you’re getting an equivalent product.”
What is the difference between kill time and product contact time?
Kill time is the time it takes a disinfectant to kill an individual pathogen or group of pathogens. Product contact time is the time it takes a disinfectant to kill all the pathogens listed on the product label. A product that kills most pathogens in one minute, but requires 5 minutes to kill a single pathogen, has a 5 minute product contact time and must remain wet on a surface for the full 5 minutes. SaniZide Pro 1® disinfectant kills all pathogens listed on the label in one minute. Therefore, SaniZide Pro 1® disinfectant has a one-minute product contact time and only needs to remain wet on the surface for one minute, saving time and increasing compliance.
Which SaniZide product is ready to use?
Both. SaniZide Plus® and Pro 1® products are both ready to use, meaning there is no diluting or mixing necessary. They can be used right from the bottle or canister.
What does “one-step clean and disinfect” mean?
A surface that is visibly soiled must be cleaned before disinfecting with any registered disinfectant. But, where a surface is not visibly soiled, only a one-step product can be used to both clean the unseen surface contamination and then disinfect the surface in a single step. Products that are not “one step” require at least two steps, and twice as much product, even if the surface is not visibly soiled.
Which SaniZide product has quicker kill times?
SaniZide Pro 1®. It is an intermediate-level EPA registered, hospital grade 1-minute surface disinfectant that contains 21% alcohol.
Which SaniZide products require you to use personal protection?
All of our SaniZide Pro 1® and SaniZide Plus® disinfectants are considered low toxicity designated by the signal word “Caution” on the label. They have this designation because they can cause moderate eye irritation. While PPE is not required, it is recommended to wash your hands thoroughly with soap and water after handling. When dealing with blood and other bodily fluids remember the proper use of disposable latex gloves, gowns, masks and eye coverings when handling soiled items or surfaces.
Are all sanitizers and disinfectants the same thing?
No. Some surface disinfectants may also have a surface sanitizer claim according to the EPA, but remember that sanitizing surfaces kills less germs than disinfecting. Surface sanitizers must also not be confused with hand sanitizers or hand wipes. Hand sanitizers cannot have specific kill claims and have not been tested on hard surfaces which means you cannot know that they will be effective on hard surfaces. Always read the product instructions for the intended use of the product.
What is the difference between cleaning, sanitizing, and disinfecting?
Cleaning removes visible dirt and other contaminants but does not kill any microorganisms. Sanitizing Kills 99.9% of Staphylococcus aureus and Klebsiella pneumoniae or Enterobacter aerogenes only, within 5 minutes. Low-level disinfection is the process of killing from 99.9%-99.999% of specific vegetative bacteria, fungi, and enveloped viruses which are listed on the product label. Intermediate-level disinfection is the process of killing from 99.9% to 99.999% of specific vegetative bacteria, fungi, enveloped viruses, and non-enveloped viruses that are listed on the product label. High-level disinfectants kill all the pathogens that are killed by intermediate-level disinfection as well as killing bacterial spores.
What is the emerging viral pathogen claim?
According to the EPA “Because the occurrence of emerging viral pathogens is less common and less predictable than established pathogens, few if any EPA-registered disinfectant product labels specify use against this category of infectious agents. Therefore, in 2016, EPA provided a voluntary, two-stage process to enable use of certain EPA-registered disinfectant products against emerging viral pathogens not identified on the product label.” To be able to make an emerging viral pathogen claim, a company must prove to the EPA that their disinfectant is effective against harder-to-kill viruses. The EPA must accept the company’s request before any claim can be made. You will hear about the emerging viral pathogen claim most often relative to SARS-CoV-2, the virus that causes COVID-19. Safetec’s SaniZide Pro 1 products, and SaniZide Plus Wipe are considered effective against SARS-CoV-2 according to the emerging viral pathogen claim.
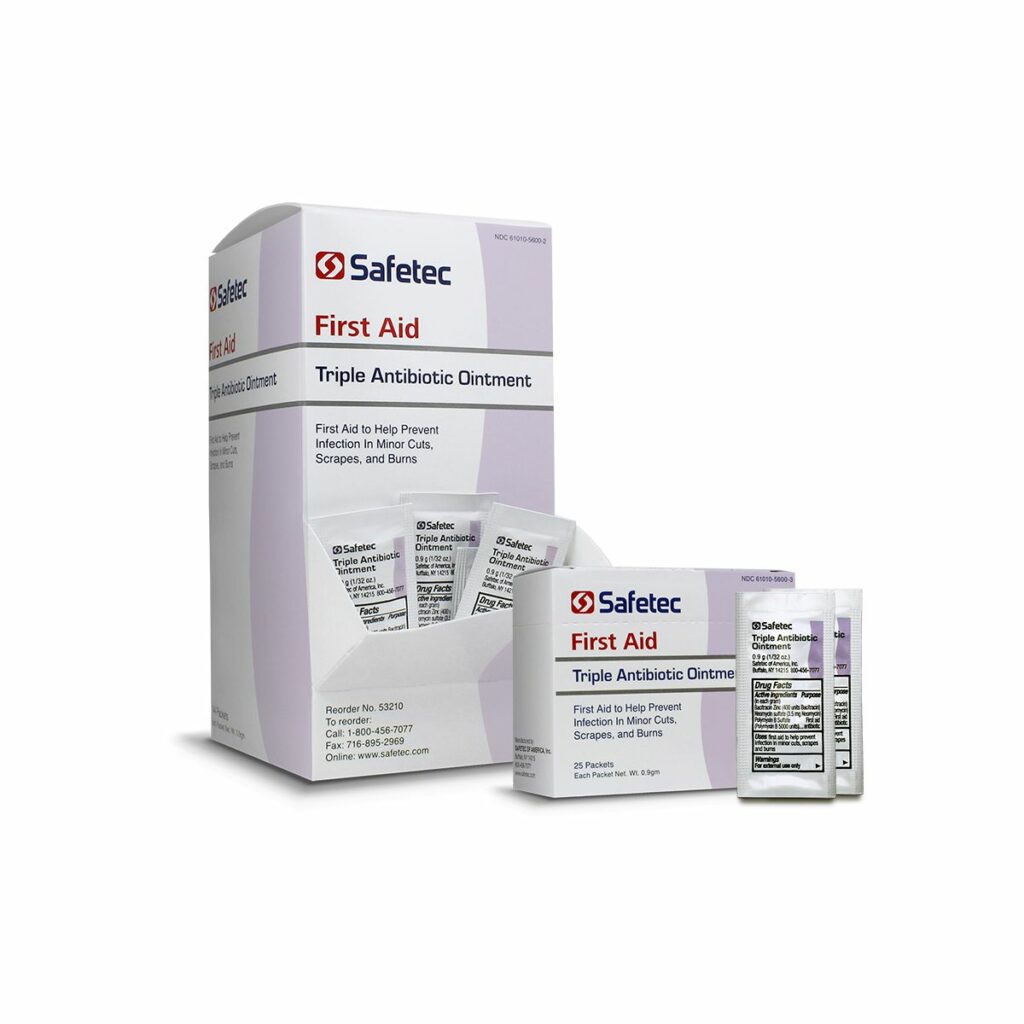
What is the difference between antiseptic and antibiotic?
Antiseptics slow the growth of a wide range of microorganisms. Microorganisms can include bacteria, viruses, and fungi. Antibiotics kill bacteria, but they are only effective against bacteria. Both options can help prevent infections on minor wounds.
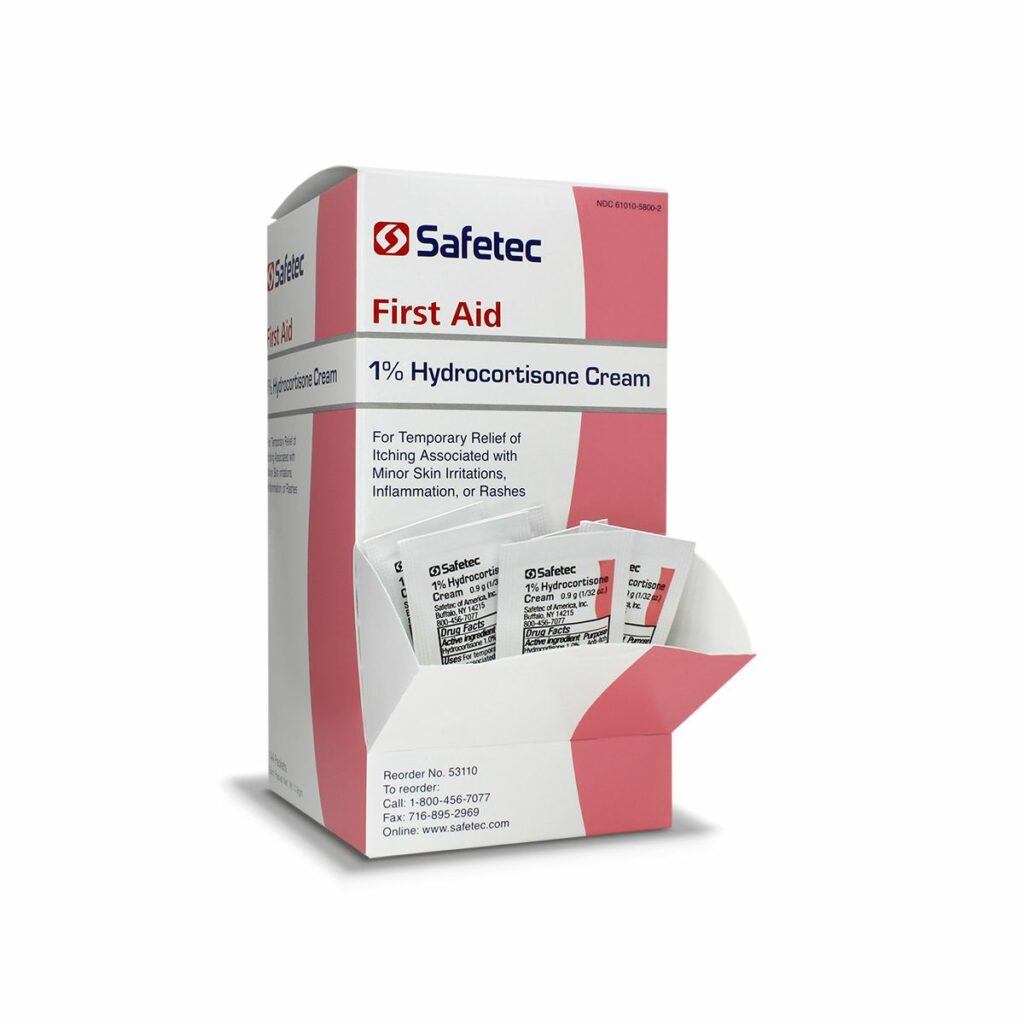
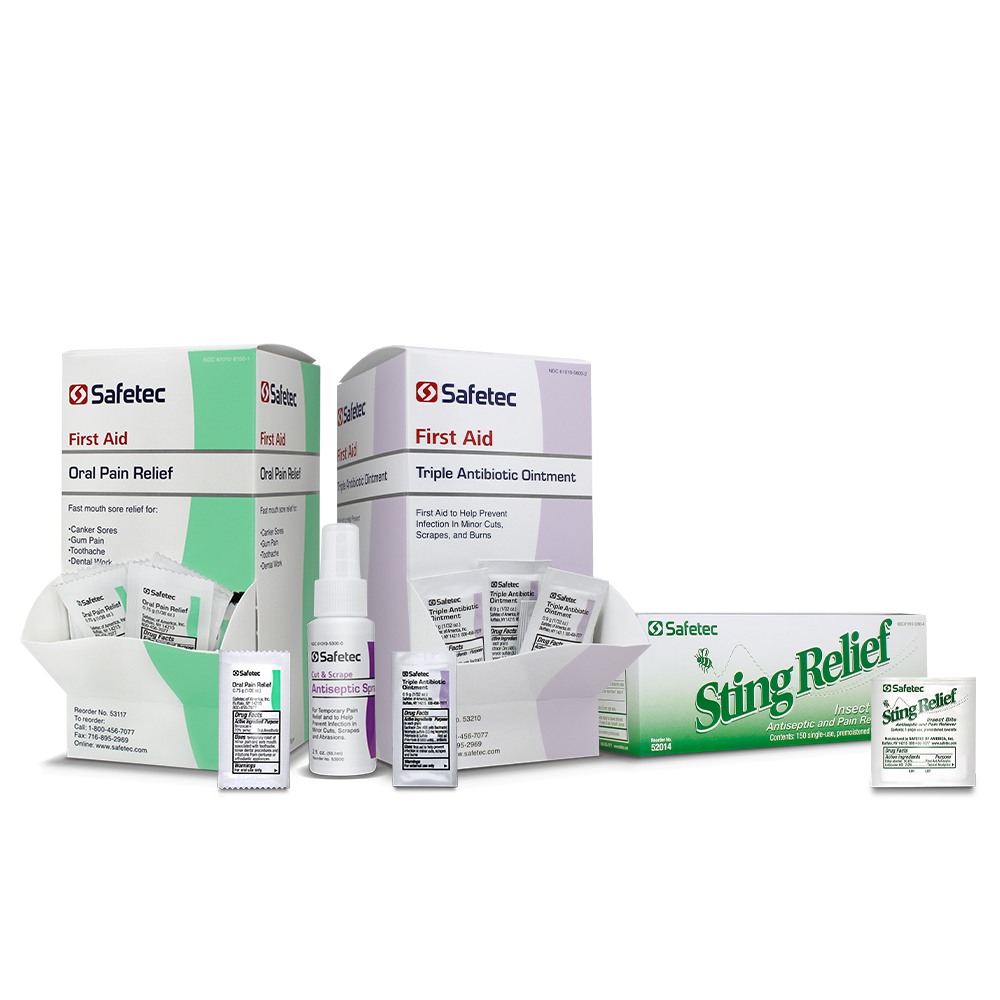
What is the difference between itch relief, sting relief, and hydrocortisone?
While some of these options can be used for similar purposes there are differences that can help you choose the right product.
• Itch Relief- Recommended for minor skin irritations and rashes due to poison ivy, poison oak, poison sumac, and insect bites. It is offered in a no touch spray which can prevent rash spreading and contains diphenhydramine (an antihistamine) as the active ingredient.
• Sting Relief- Recommended for temporary relief from pain and itching caused by insect stings or bites. It is available in a no touch spray, or as individually packaged wipes. Sting relief contains lidocaine (topical pain reliever) and ethyl alcohol (first aid antiseptic) as active ingredients.
• Hydrocortisone (1%) Cream- Recommended to prevent itching from minor skin irritations, inflammation, or rashes due to eczema, poison ivy, dermatitis and more. This is a cream product available in tubes and individual pouches. The active ingredient is hydrocortisone which is a corticosteroid used to reduce swelling.
How do I find my lot number and expiration date?
Pouches: Both are stamped near the bottom of the product on the back, with the first 7 numbers showing the expiration date and 4 digits showing the lot number (ex. YYYYMMM LNNN). Bottles: Both are laser printed near the bottom of the product, either right on the bottle or can be found on a sticker on the bottle. Boxes: Both are found either on a sticker on the box or laser printed.
Why does my product not have an expiration date on it?
Safetec Kits: The outer packaging of any Safetec kit does not receive expiration dating during manufacturing because there are multiple items within a kit that each have different expiration dates, depending on the individual date of manufacture. Safetec does strive to efficiently package kits with only the most recently manufactured internal components, ensuring a 2 to 3-year shelf life. OTC products (have Drug Facts): Over-The-Counter products that do not have a dosage limit (like amount per day) do not require expiration dating. Safetec is required however to have proven test studies showing product stability over a 3-year shelf life, by the FDA. If a product states a dosage limit in the Drug Facts-Uses like “not more than 3 to 4 times daily” then an expiration date will be displayed on the innermost packaging.
Why do you have different types of antibiotic ointments?
Some people are allergic to certain types of antibiotics. By offering multiple options we hope that we can provide you and your customers an option for everyone!
How do I find my lot number and expiration date?
Pouches: Both are stamped near the bottom of the product on the back, with the first 7 numbers showing the expiration date and 4 digits showing the lot number (ex. YYYYMMM LNNN). Bottles: Both are laser printed near the bottom of the product, either right on the bottle or can be found on a sticker on the bottle. Boxes: Both are found either on a sticker on the box or laser printed.
Why does my product not have an expiration date on it?
Safetec Kits: The outer packaging of any Safetec kit does not receive expiration dating during manufacturing because there are multiple items within a kit that each have different expiration dates, depending on the individual date of manufacture. Safetec does strive to efficiently package kits with only the most recently manufactured internal components, ensuring a 2 to 3-year shelf life. OTC products (have Drug Facts): Over-The-Counter products that do not have a dosage limit (like amount per day) do not require expiration dating. Safetec is required however to have proven test studies showing product stability over a 3-year shelf life, by the FDA. If a product states a dosage limit in the Drug Facts-Uses like “not more than 3 to 4 times daily” then an expiration date will be displayed on the innermost packaging.
Distributor Resources
View All
How To Properly Handle A Hazardous Drug Spill

Product Catalog
Downloads
Product Safety Data Sheets
Product Sell Sheets
Compliance Documents